Written by: Manuel Contreras, DVM, MS, Diplomate ACPV, Agrimprove/Special Nutrients
KEY TAKEAWAYS:
- Endotoxins are only present in Gram-negative bacteria.
- Endotoxins can affect commercial poultry when some bacterins are injected without following the manufacturer’s instructions.
- There are not many scientific reports currently reporting impaired performance, lesions, and symptoms associated with the presence of endotoxins in Poultry.
The importance of endotoxins in poultry production is a new topic frequently covered in animal production and veterinary medicine technical magazines worldwide. Marketing campaigns are, in part, responsible for this trend. Some commercial/technical articles show the effect of several mycotoxin binders against endotoxins. Reviewing some of these reports, we quickly detected the absence of measurements indicating that endotoxins were present in the evaluated animals. Therefore, it is worth asking how we can associate performance improvements when using these additives if, during the trials, the presence or reduction of endotoxins was not reported. We fully understand that a significant limitation in detecting endotoxins is the lack of availability of reliable laboratory tests in blood, urine or feces. So far, the techniques available are inaccurate, and the interpretation of the results tends to confuse researchers. In the last decade, we have evaluated the results of field tests carried out in pig farms in the European Union, where endotoxins were suspected of causing production deficiencies. In this case, a mycotoxin binder was evaluated to ameliorate the poor performance reported.
Although a statistically significant effect was reported in various production parameters when using the additive, it was not clearly established that its inclusion in the feed caused the changes.
What are endotoxins?
Endotoxins are used to describe a complex containing lipopolysaccharides (LPS) associated with the outer membrane of bacteria such as Escherichia coli, Salmonella, Pseudomonas, and Pasteurella.
Gram Negative bacteria have a cell envelope that contains three layers or membranes:
- Cytoplasmic membrane (interior)
- Peptidoglycan membrane or R layer
- Outer membrane
Since Gram-positive bacteria do not have an outer membrane, endotoxins are not present.
How are endotoxins released?
1. Production during the initial bacterial growth phase, both in the laboratory (in vitro) and in animals (in vivo).
Through this mechanism, minimal amounts of soluble endotoxins are released. It is important to emphasize that for this growth and release to occur, a liquid medium is needed. Once released from the cell wall, an immune response is initiated that depends on the type and concentration of LPS, duration of exposure, host genetics, and the presence of clinical signs caused by viral or bacterial infections.
2. Destruction (lysis) of bacteria by the immune system or antimicrobial agents.
Since the intestines are loaded with Gram-negative bacteria, they represent animals’ most significant source of endotoxins. Its elimination in the feces allows it to combine with food and form a bioaerosol that can stimulate an inflammatory response in the respiratory tract. In experimental tests, when performing intra-tracheal inoculation of endotoxins in broilers, hypertension of the lungs was reported, and it is speculated that it may play an essential role in the development of ascites.
To emphasize the excellent dissemination capacity of this bio-aerosol in commercial poultry production, the same type of endotoxin present in birds has been detected in the blood of farm personnel.
The most important bacteria containing endotoxins belong to the group Enterobacteriaceae, which inhabits the normal intestinal microflora of birds and mammals (including humans).
Composition of LPS:
- Lipid A
- Nucleus
- O antigen
Lipid A is a hydrophobic structure not mixed with water and is associated with toxicity. It acts as an anchor when bacteria invade the host’s cells. Even though endotoxins differ, Lipid A is always the same regardless of the bacteria. This factor explains why endotoxins from different bacteria cause the same type of damage in the host.
When animals are exposed to a stressful environment, the concentration of free endotoxins in the body increases. Once the balance established in the intestinal microflora is lost (dysbacteriosis or dysbiosis), the result is the development of Salmonellosis and/or Colibacillosis. In other words, the absence of some beneficial bacteria, such as Lactobacillus, will allow pathogenic bacteria to grow in the intestines.
It is essential to differentiate endotoxins from exotoxins. Gram-negative and Gram-positive bacteria produce the latter and mainly affect the host. Unlike endotoxins, exotoxins are secreted by bacteria in small amounts and are lethal.
Can endotoxins cause harm to commercial birds?
Deleterious effects have been observed when injecting bacterins prepared with Gram-negative bacteria for decades. Scientific publications have shown that by injecting small concentrations of endotoxins from Pasteurella multocida in broilers, the clinical signs of acute cholera can be reproduced without the need to challenge using the whole bacteria. The best example of this type of damage at a commercial level is caused by the injection of bacterins against Fowl Cholera, which depressed birds characterize after its application in pullets during the rearing period.
On the other hand, the reactions at the application site are significant due to condemnations at the slaughterhouses, especially when using oil emulsions. A recommendation by biological companies that illustrates the importance of endotoxins when vaccinating is to keep vaccines at an average temperature of approximately 37 ºC. Overheating will release the endotoxins present in the vaccine, and mortality and hemorrhagic syndrome could occur.
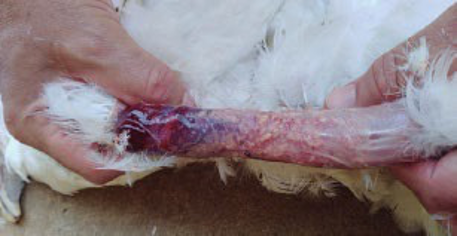
Photo 1. Local reaction at the application site of a bacterin injected into the neck of a 12-week-old commercial pullet, probably caused by endotoxins (LPS). The photo was taken at 18 weeks and shows yellowish caseous material of bacterial origin due to needle or syringe contamination.
How are endotoxins prevented?
Determining if they are harming birds before investing in prevention is critical. The following strategies can be used if a real deleterious effect is established.
- Vaccination with endotoxin segments. Lipid A has been used experimentally to obtain protection. The big drawback is the high cost of these products.
- Use of mycotoxin binders mixed with the feed (organoclays). In in–vitro tests, about 90% adsorption capacity against endotoxins is reported. However, this does not mean they necessarily work when used in animal feed. Several scientific tests conducted with pigs in the United States, challenged with pathogenic strains of E. coli, have demonstrated the efficacy of some clays in reducing the incidence of diarrhea and poor performance. Although endotoxins are not measured in these reports, it is speculated that part of the efficacy of these products is a consequence of neutralizing the endotoxins released by the bacteria used to challenge.
- It is critical to follow the manufacturer’s recommendations when applying bacterins to prevent the impact of endotoxins.
In conclusion, although the adverse effects caused by endotoxins in pigs and dairy cows seem to be more established, their negative impact on poultry is not categorically demonstrated.



