Written by: Josep Garcia-Sirera, Special Nutrients Agrimprove
KEY TAKEAWAYS:
- Endotoxins have a wide variety of effects on livestock and negatively affect production performance.
- Different methods of endotoxin mitigation have been tested, but most are too expensive to be considered in animal production.
- A more cost-effective method would be using toxin binders to capture endotoxins in the gastrointestinal tract and prevent them from entering the blood systems.
What are Endotoxins?
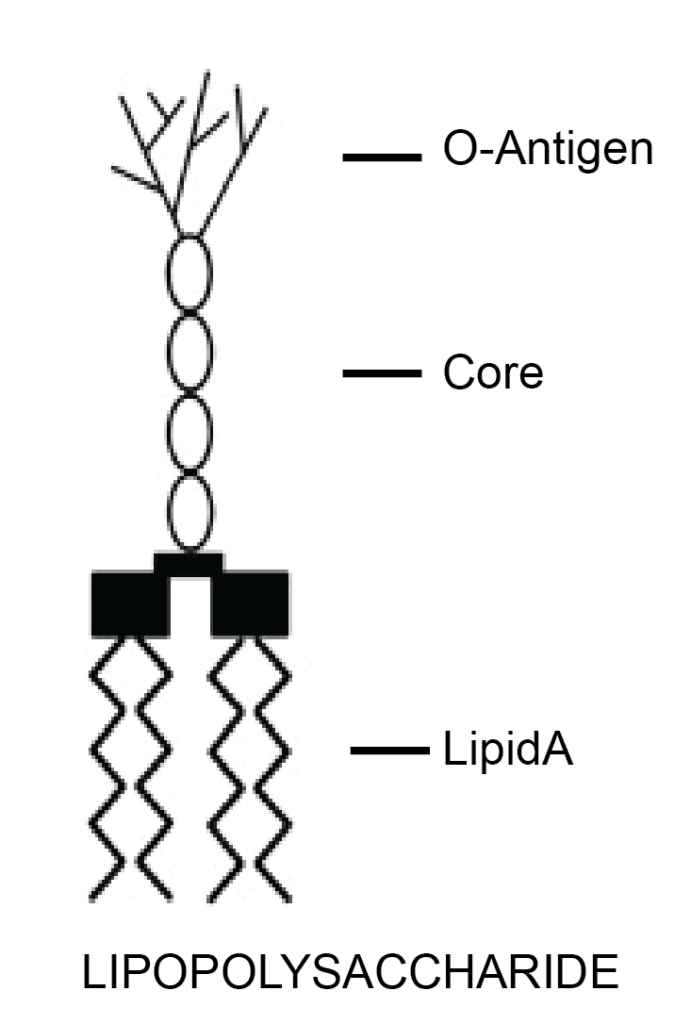
Gram-positive and Gram-negative bacteria are differentiated, among other things, by their type of cell envelope. Gram-negative bacteria are encircled by a thin peptidoglycan cell wall that is also enclosed by an outer membrane containing lipopolysaccharides (LPS), also called endotoxins. Gram-positive bacteria do not have an outer membrane; therefore, endotoxins are absent in this group. Gram-negative bacteria have a cell envelope that contains three essential layers or membranes: cytoplasmic (inner), peptidoglycan or R-layer, and the outer membrane. The latter contains phospholipids, proteins, and LPS. On the other hand, LPS consists of three elements: Lipid A, a hydrophobic component that serves as an anchor when a bacterium invades a host’s cell. The core is an oligosaccharide, and the O antigen is a hydrophilic component. Lipid A is apparently responsible for most of the toxicity caused by endotoxins.
Figure 1. Differences between Gram-negative and Gram-positive bacteria
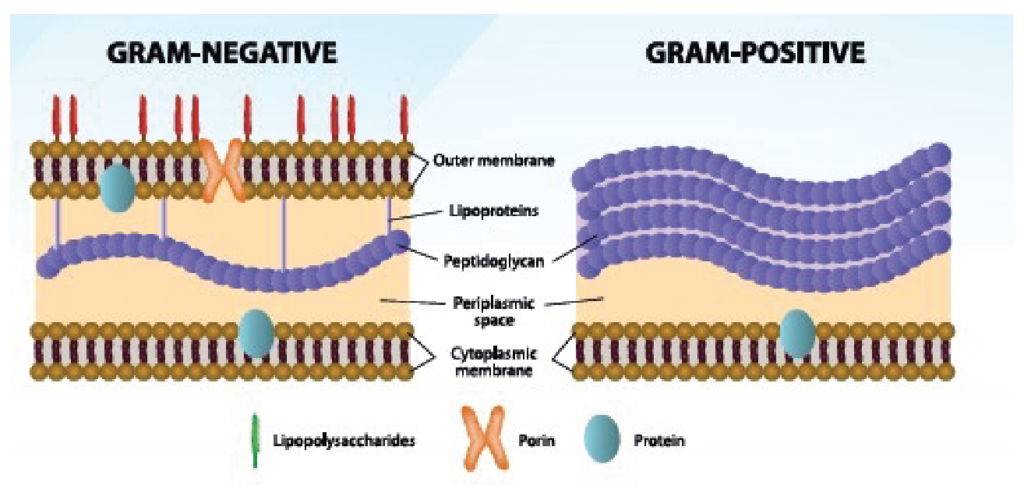
Figure 2. Structure of LPS or endotoxin
Endotoxins are common in the gastrointestinal tract (GIT) of animals, particularly in the large intestine. They are also present in bioaerosols that produce inflammatory reactions, particularly in the respiratory tract. It has been detected that bioaerosols, coming from feed, litter, and feces, have the highest concentration of bacterial endotoxins. When endotoxins are present in the GIT and excreted together with feces, these endotoxins will be attached to the dust particles present in the farm and end up in the air, which causes the inhalation of particles by the animals. As a result, we can observe respiratory or gastrointestinal diseases affecting the animals that inhale them.
Under normal commercial production conditions, a small number of endotoxins will be transferred from the GIT to the bloodstream. According to scientific papers, it represents concentrations lower than ten pg/ml (picograms per milliliter), and these levels are capable of stimulating the immune system. Under stress, the level of free endotoxin in the GIT will be higher. Stress can produce dysbiosis or dysbacteriosis, that is, microbial imbalance in the intestines and the production of more endotoxins in the system. It is critical to emphasize the importance of keeping the correct microbial balance in the intestines because diseases such as Salmonellosis or Colibacillosis are intrinsically associated with the unrestricted growth of pathogenic bacteria that displace the intestinal favorable microflora (i.e., Lactobacillus to mention).
When bacteria are eliminated due to the administration of antimicrobials or because of the work of the immune system, bacterial cells will be destroyed, and the final consequence is the liberation of endotoxins that will harm the animal. Endotoxins are released from the bacterial cell wall during the growth and division phases of the microbe.
Understanding the Mode of Action
The mechanism is complex. In humans, LPS binds to a lipid binding protein (LBP) that moves LPS to the serum to eventually bind with Toll-like receptor-4 (TLR4). This triggers the signaling cascade for macrophage/endothelial cells to secrete pro-inflammatory cytokines and nitric oxide that leads to characteristic “endotoxic shock.”
The injection of living or killed gram-negative cells or purified LPS into experimental animals causes a wide spectrum of nonspecific pathophysiological reactions, such as fever, changes in white blood cell counts, disseminated intravascular coagulation, hypotension, shock, and death. Injection of small doses of endotoxin results in death in most mammals. The sequence of events follows a regular pattern: (1) latent period; (2) physiological distress (diarrhea, prostration, shock); and (3) death. How soon death occurs varies depending on the dose of the endotoxin, route of administration, and animal species. Animals vary in their susceptibility to endotoxin.
Impact on the pig

The effect on production that comes from exposure to endotoxins in swine is a consequence of two factors (Parra et al., 2011):
- The inflammatory response translates into alterations in the gastrointestinal barrier, blood circulation, fever, and other symptoms. In 2011, Parra S et al. studied the effects of endotoxins on the morphology of the gastrointestinal gut barrier. Results showed that LPS decreased the height and area of the intestinal villi and increased the width of the villi and the depth and width of the intestinal glands. The authors concluded that these effects probably could contribute to decreased intestinal nutrient absorption and increased co-infection with other pathogens, thus leading to post-weaning diarrhea syndrome. Fever is mediated through the action of IL-6, known to be increased because of exposure to endotoxins. Other symptoms that can be related, even though they have multifactorial causes, would be ear necrosis and lesions that lead to tail biting.
- There is a nutrient expense derived from the need for the inflammatory system to be activated. As a result of inflammation, endotoxemia leads, among other things, to a feverish state that results in reduced feed intake. The productive performance of farm animals (i.e., producing milk, eggs, meat, etc.) requires energy and shows a poor feed conversion rate (FCR). The energy required for the inflammatory response will come from the energy used normally for production. This results in lower FCR and decreased growth performance.
J. Parra et al., Lipopolysaccharide (LPS) from E. coli has detrimental effects on the intestinal morphology of weaned pigs. Rev Colom Cienc Pecua Vol.24 no.4 Medellín Oct./Dec. 2011.
Controlling Endotoxins
In general, strategies to control endotoxin contamination in animals are aimed at the reduction of bacterial contamination. These strategies include biosecurity, prebiotics, probiotics, improved nutrient digestibility, etc. Other strategies include vaccination, immunomodulation, and the use of toxin binders scientifically proven to target endotoxins.
Vaccination: Currently, immunization against Lipid A is under development, but the high cost makes it a non-viable option for livestock production. Another option considered in vaccination is to immunize against LBP. By neutralizing the reaction of the LBS-LBP complex, the cascade of events leading to pathogenesis can be reduced. This option is also expensive and is currently only applied for human use.
Immunity modulators: The use of immune modulators to compensate for the effects of endotoxins has been tested in animal production. An example of this immune-modulating action is found in B-glucans present in the yeast cell wall, which can reduce LPS-induced inflammation, though it does not prevent inflammation.
Toxin binders: A more practical approach to reduce the absorption of endotoxins from the gastrointestinal tract of livestock is the use of toxin binders. Toxin binders are widely used to control other toxins, such as mycotoxins. The binder and the mycotoxin form a complex that is too large to be absorbed into the blood system. The complex is then eliminated in the feces. Most mycotoxin binders are hydrophilic molecules (ex., bentonites, aluminosilicates, etc.) efficient at capturing polar molecules such as Aflatoxin. The capacity of these traditional mycotoxin binders to capture more lipophilic-like molecules such as zearalenone or DON is questionable.
Some preliminary studies have already tested the capacity of different toxic binders against endotoxins in vitro. A recent study at the University of Ghent went beyond in-vitro data and effectively proved the capacity of a toxin binder to capture endotoxins in the intestines of piglets. In the study, the authors compared the effect on the production of cytokines by the injection of endotoxins, with or without the addition of the toxin binder. The model was intestinal loops of live piglets.
The data proved that the toxin binder was capable of reducing the cytokine production that results from endotoxin activation of the receptor.
Summary
Endotoxins have a wide variety of effects on livestock, affecting performance parameters. Different means of control of endotoxins have been tested, but most of them are too expensive to be considered in animal production. A cost-effective method would be the use of toxin binders to capture endotoxins in the gastrointestinal tract and prevent them from entering the blood systems. Some studies are underway to test this possibility.

